Newsroom

Announcements
Announcements
-
Board & Staff
December 16, 2025
ADDF Bolsters Leadership Amid Transformative Moment in Alzheimer’s Research, Naming Gavin Cook and David Dolby to its Board and Appointing Jane McIntosh as Interim CEO
-
Research Update
December 4, 2025
New Data from Semaglutide Trials Provides Critical Insights to Guide Next Generation of Therapies Targeting Alzheimer’s Pathobiology
-
Research Update
November 24, 2025
Readout of Phase 3 Semaglutide Trials Marks Critical Moment in Alzheimer’s Research and Suggests Potential for Combination Therapies
Alzheimer's Matters Blog


Developing Drugs
December 2, 2022
CTAD Showcases ADDF Leadership and Historic Progress in Alzheimer’s Research
The 2022 Clinical Trials on Alzheimer’s Disease (CTAD) conference has been a truly historic meeting, and the Alzheimer’s Drug Discovery Foundation (ADDF)’s role in bringing about a new era of research was evident throughout.

ADDF Impact
October 17, 2022
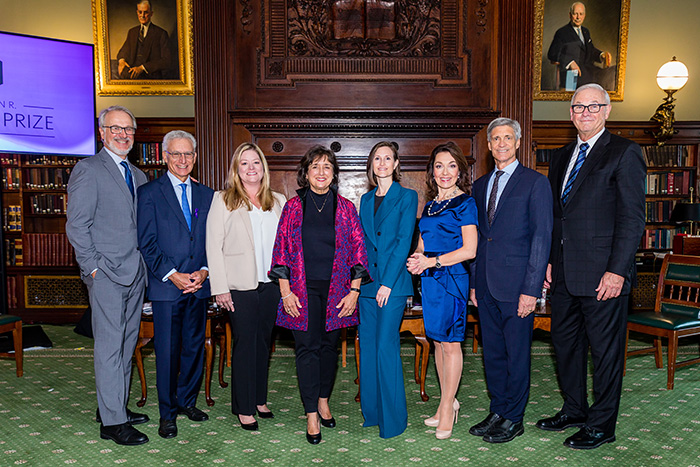
Celebrating Alzheimer’s Science and Scientists at the 2022 ADDF Goodes Prize Luncheon
Five previous winners of the prestigious Melvin R. Goodes Prize came together in New York City recently to honor this year’s winner, Miranda E. Orr, PhD, Assistant Professor of Gerontology and Geriatric Medicine at the Wake Forest University School of Medicine, and to discuss how their research is working toward the same goal – to conquer Alzheimer’s.


Protecting Brain Health
May 31, 2022
Can Lifestyle Interventions Combined with a Diabetes Drug Prevent Cognitive Decline?
Alzheimer’s Drug Discovery Foundation Funds Second Phase of Global Alzheimer’s Prevention FINGER TRIAL
Resources
Featured Video
On April 9, the ADDF celebrated its second annual Memories Matter benefit event at Chelsea Piers, which welcome nearly 600 guests and raised over $1 million for Alzheimer's research.
View more on our YouTube channel