On Wednesday, promising findings from a small phase 1 clinical trial of the potential Alzheimer’s treatment aducanumab were published in the journal Nature. For the first time, a treatment has reduced beta-amyloid plaques in the brains of patients with Alzheimer’s disease. The trial results excited those of us working to find new treatments for Alzheimer’s and provided hope to tens of millions of people living with this disease.
Aducanumab is a monoclonal antibody vaccine being developed by Biogen that is given intravenously once a month. Patients in the trial who received the treatment had fewer beta-amyloid plaques in their brains and slower cognitive decline than patients receiving a placebo. And those effects were greater in patients receiving higher doses, meaning the benefits were likely due to the treatment and not chance. In a commentary accompanying the journal article, Eric M. Reiman, executive director of the Banner Alzheimer’s Institute called the results: “a major advance.”
This was one of the first clinical trials to ensure that every patient participating had beta-amyloid plaques in their brains. While it seems obvious that you’d want to make sure patients had such plaques if a treatment aims to remove them, that hasn’t been the case. Researchers have instead used tests that measure “downstream” effects such as memory problems as criteria to enroll patients. Studies have indicated that up to 30 percent of those enrolled in previous clinical trials didn’t in fact have plaques and possibly didn’t have Alzheimer’s at all. This is likely a factor in why 99.6% of all treatments in late stage clinical trials for Alzheimer’s disease have failed.
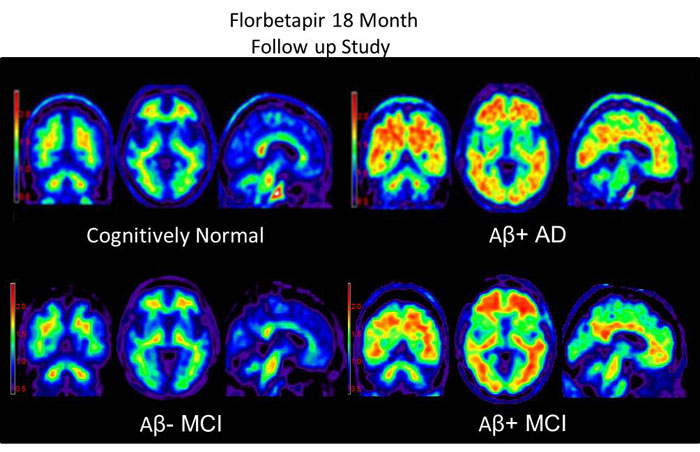
Until a few years ago, you couldn’t conclusively diagnose Alzheimer’s in living patients. That all changed in 2012 when the FDA approved Amyvid™ (florbetapir), the first diagnostic test for Alzheimer's disease. This PET scan images beta-amyloid plaques in the brain. The team conducting the aducanumab clinical trial used Amyvid™ to ensure that all patients enrolled in the trial had beta-amyloid plaques and to assess the effectiveness of the treatment at different doses in removing those plaques.
This breakthrough was made possible through the support of the ADDF. We funded the development of the Amyvid™ scan from 2000–2004, allowing its creators to advance their idea to a stage at which they could attract more investment and ultimately win FDA approval. This single PET scan has now enabled the most promising results ever seen for an Alzheimer’s treatment.
We hope these results encourage more researchers developing treatments that target plaques to use the Amyvid™ scan. At the ADDF, we fund drugs that target the diverse causes of Alzheimer’s, from neuroinflammation and vascular disorders to tau tangles, genetic risk factors, epigenetic changes, and mitochondrial dysfunction. And we are supporting diagnostic tools to ensure that clinical trials will be able to accurately assess whether patients actually have these underlying causes and how various treatments affect them. As aducanumab has demonstrated, these diagnostic tools can mean the difference between success and failure in a clinical trial.
Aducanumab is now being tested in two large-scale phase 3 clinical trials that are scheduled for completion in 2020. If its positive results hold, it will be a game-changer for the treatment of patients with Alzheimer’s disease.
Howard Fillit, MD is the Founding Executive Director and Chief Science Officer at the ADDF.